
by Laura Wallis
Before any scientific question can be answered, it must be dreamed up. What happens to cause a healthy cell or tissue to change, for instance, isn’t fully understood. While much is known about chemical exposures that can lead to genetic mutation, damaged DNA, inflammation, and even cancer; what has rarely been asked is how physical stressors in the environment can cause a cell or tissue to respond and adapt. It’s a piece of the puzzle upon which future medical breakthroughs might depend.

Alison Patteson (left) and Jennifer Schwarz (right), both professors in the Department of Physics and members of the BioInspired Institute, have been awarded a four-year National Science Foundation grant from Physics of Living Systems, for a project titled Mechanical Homeostasis—an Emergent Property of the Multi-Tiered Structure of Living Cells and Tissues.
Homeostasis refers to a state of equilibrium; at the cellular and tissue level, any changes in environment will spur a response that balances or accommodates it. “Mostly people think of chemical changes, exposure to drugs, for instance,” says Schwarz, principal investigator on the project. “Here we ask, what if you squeeze a cell—or a group of cells or tissue—mechanically? Can it still carry out its functions? Maybe not. Maybe it needs to adapt.”
As co-principal investigator Patteson notes, describing the idea this way is a new use of scientific language. “As physicists, we are proposing this idea that there is a mechanical version of homeostasis,” she says. “We have proposed a framework for that.”
Drawing upon previous collaborations that have examined specific scales (such as chromatin molecules, individual cell motion, and collective cell migration through collagen networks), the investigators will work to build a multiscale model to capture how chromatin remodels from physical stressors at the cell- and tissue-level. They will conduct experiments involving mechanical compression, and working with the Blatt BioImaging Center, observe detailed microscopic images of the cells in action.
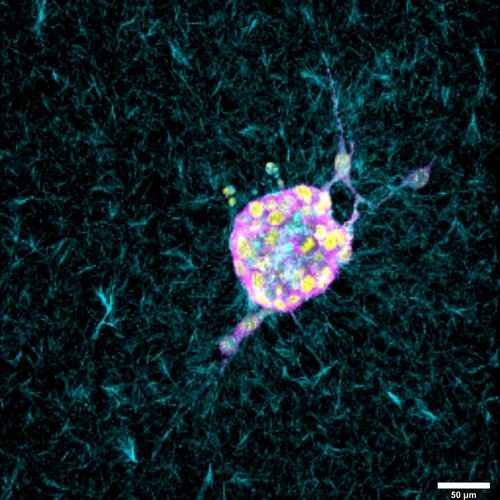
Understanding these mechanisms may have broad implications in health research, shedding light on the causes of and therapeutic treatments for inflammation and potentially, cancer.
“We know that most cancerous tissues get stiffer,” notes Patteson. “That’s how you identify it. There’s clearly a change in mechanics associated with the development of the disease.”
But much remains to be discovered about the interactions and processes at different scales. “We’re not at therapeutic levels yet,” says Schwarz.
The professors note that creativity is essential to this stage of research—in imagining what might be possible and what new questions to ask, and in pushing the boundaries of existing scientific language. To that end, they have incorporated broader outreach between the physics and creative writing departments in their project.
In a collaboration with creative writing professors Sarah Harwell and Jonathan Dee, along with M.F.A. candidate Maria Marchinkoski, students from both departments will cross over and embed in their respective classes. “[They’ll see] how a piece of poetry is creative, for example. Then, how a certain experiment is creative,” says Schwarz. “We want to get physicists thinking like creative writers, and vice versa.”
The colleagues like to think that students and their work will benefit from the exercise, not only in expanding their ideas of what is possible but also in taking a more thoughtful approach to the language they use. Instead of talking about hierarchy of scales,” says Patteson, “maybe we should be talking about coupled things, or partnerships.” A simple shift in perspective, after all, can sometimes put things in a whole new light.
