Protein Droplets: A New Way to Understand Disease
Key Takeaways:
- Powerful protein droplets: Our cells use tiny droplets to manage broken or damaged proteins. When this system does not work well—such as when we get older—proteins can pile up and may lead to brain disorders like Alzheimer’s and ALS.
- Acting on Damaged Proteins: Droplets show up in cells when needed depending on stress, temperature or other signals. Scientists want to learn how these droplets know when to form and which proteins to fix or discard.
- Developing Droplets: Syracuse University scientists are building artificial droplets in the lab and watching real droplets in cells to better understand how they work—and how they might help stop diseases.
When we are young and healthy, our cells successfully monitor and manage our worn-out or damaged proteins, keeping things working properly. But as we age, this cleanup system can falter, leading to protein clumps linked to neurodegenerative diseases such as Alzheimer’s disease and ALS (amyotrophic lateral sclerosis). Now Syracuse University scientists are diving deep to understand how these tiny, temporary droplets–known as condensates–work, which could lead to new ways of treating or preventing several brain disorders.
Aging is tough on protein management in our cells. “The mechanisms that we call protein quality control do not work as well anymore,” says Carlos Castañeda, associate professor of biology and chemistry in the College of Arts and Sciences. Castaneda has been awarded a five-year, $2 million National Institutes of Health R35 MIRA award to study the link between protein quality control and “biomolecular condensates.”

Carlos Castañeda
“Losing protein quality control is related to some neurodegenerative disorders,” says Castañeda. “We are trying to understand those mechanisms so we can see why cells are not able to take care of proteins as they did earlier in life.”
Storage Closets and Trash Dumps
Scientists are discovering that cells contain tiny droplets that function like liquid storage closets, gathering, fixing, recycling or removing dysfunctional proteins. But as we age or respond to stress, our cells can lose effectiveness in cleaning up and managing these proteins.
When repair and recycling systems are lacking, damaged proteins can accumulate, forming clumps that may contribute to neurodegenerative diseases like Alzheimer’s disease and ALS. The droplets themselves can harden into sticky protein clumps, leaving long-term trash dumps in the brain.
In recent years, scientists have learned that droplet compartments are not rigid, permanent parts of the cell. Instead, they are membrane-less gatherings of specialized proteins that cluster together under certain conditions. These droplets appear and disappear when needed, helping cells adapt. Droplets gather and disperse based on stress, temperature and cellular signals.
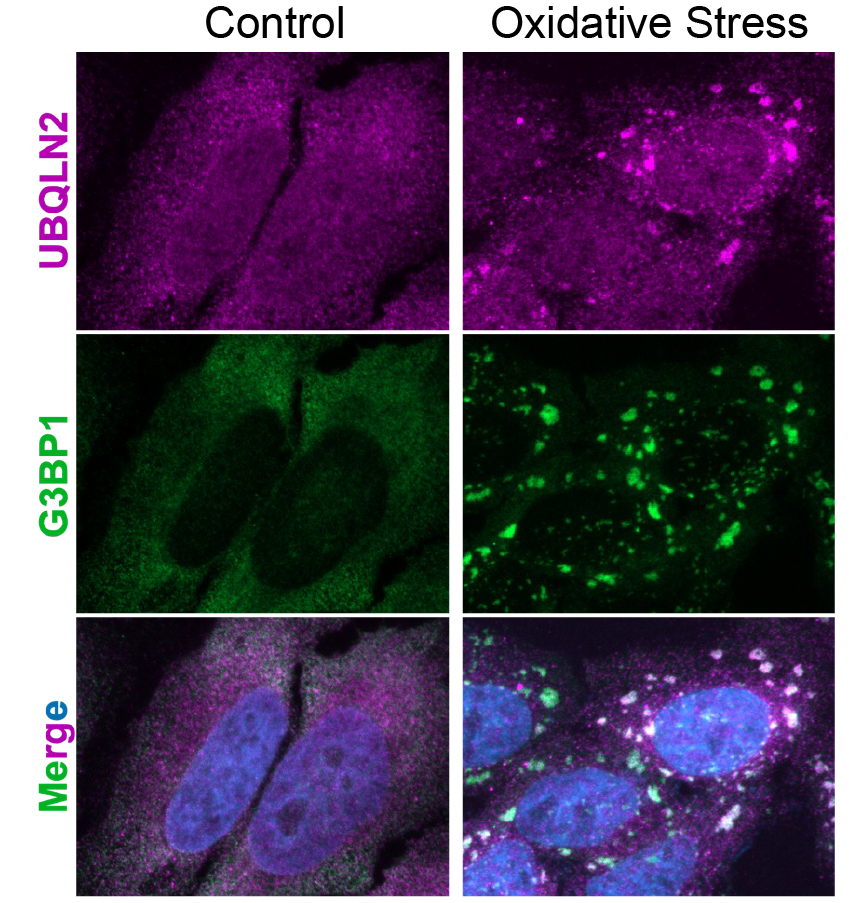
The Castañeda team aims to learn more about what causes droplets to form, what droplets are made of, and how droplets decide which proteins are problematic and need fixing, recycling or removing.
Forces at Work
The research team will use a dual approach. They will perform molecular experiments to learn about changes to protein structure and dynamics, and cell biology-based approaches to observe living processes.
In molecular work, they will construct artificial droplets outside of cells to watch how changes in protein combinations or stress signals change their behavior, such as their ability to recruit different proteins or mediate different downstream outcomes (protein degradation or not).
The team will also perform studies of living cells. The researchers want to know more about how droplets manage damaged proteins when cells are stressed. They will study cellular signals that form these droplets and how different protein combinations can affect droplet behavior.
“We make a droplet in a test tube to see how the organization of these components change with different conditions and take components apart so we can understand how they come together,” says Castañeda. “Think of it as understanding a car engine by both building and dismantling it.”
These basic scientific investigations could have transformative long-term impacts, such as identifying critical points where intervention might prevent or treat protein clumps. It could potentially illuminate similar mechanisms across different neurodegenerative disorders and other diseases such as cancer.
Syracuse University's collaborative and supportive research ecosystem (e.g., the Bioinspired Institute, the Bioimaging Center, high-field NMR at SUNY-ESF) has been crucial to the development of this study, allowing scientists in different fields to share techniques and insights, access specialized equipment and develop more comprehensive research strategies, Castañeda notes.
“This field requires scientists from multiple fields—biology, chemistry, physics and engineering—working together,” says Castañeda. “This work would not have been possible without the many talented postdocs, graduate students, undergraduates, and high school students that have gone through our lab. A special thanks to our lab manager and senior scientist Dr. Thuy Dao. I am deeply appreciative of our key collaborators at SU (e.g., Heidi Hehnly, Shahar Sukenik, Heather Meyer, Li-En Jao) and beyond (Dan Kraut at Villanova, Jeroen Roelofs at KUMC). Finally, I am very grateful to A&S and the VPR office for their support over the years.”
Published: Aug. 7, 2025
Media Contact: asnews@syr.edu
