
James Kallmerten
- Chemistry
Research Interests
Organic chemistry; natural products; synthesis; electro-optical materials
Education
- B.Sc., 1975, Massachusetts Institute of Technology
- Ph.D., 1979, Brown University
- Postdoctoral Fellow, 1979-1981, Columbia University
Honors & Awards:
- National Institutes of Health Postdoctoral Fellowship, 1979-1981
- Lilly Distinguished Teaching Fellowship, Chancellor's Citation, American
- Cyanamid Academic Award, Teacher-Scholar of the Year (1998)
Courses
- CHE 275: Organic Chemistry I
- CHE 325: Organic Chemistry II
- CHE 427/627: Organic Chemistry of Biological Molecules
- CHE 686: Advanced Organic Synthesis: Design
Research Focus
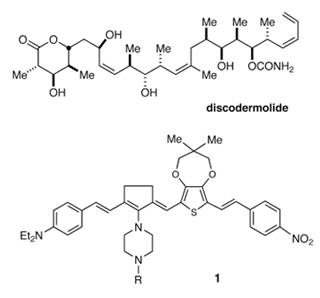
The emphasis of our research program is the development and implementation of new synthetic methods and strategies for the synthesis of biologically active, naturally occurring compounds. The rationally designed synthesis of a complex molecule serves as a critical test of our current understanding of structure and reactivity, often illuminating the strengths and deficiencies of existing methodology. Total synthesis is often the only means of obtaining the quantities of biologically active substances required for clinical evaluation, and offers a viable route to modified derivatives with potentially enhanced therapeutic indices. Compounds under investigation include herbimycin A, an anti-tumor ansamycin which inhibits oncogene-derived protein tyrosine kinases, the powerful immunosuppresive agents rapamycin and discodermolide. Our synthetic studies will provide a reliable source of these materials to support preclinical studies and new analogs for use as probes of the biological mechanism(s) which underlie the anti-tumor activity of the parent compounds.
Supporting our synthetic studies are basic methodological investigations designed to uncover new and more effective tools for the construction of complex organic materials. Of particular interest to my group is the potential of stereoselective sigmatropic reactions such as the Claisen and [2,3] Wittig rearrangements to establish the stereochemically intricate carbon frameworks of acyclic and macrocyclic compounds of biological interest. A long-range goal of this program is the incorporation of these and other stereoselective sigmatropic variants into an efficient and comprehensive strategy for the synthesis of polyketide-derived natural products.
A final area of current interest is the design and synthesis of labeled probes and other small organic molecules for use in the study of fundamental chemical problems. Of current interest is the design and synthesis of new organic materials with electro-optical properties. Compounds such as 1 have potential applications to problems in telecommunications and molecular electronics.
Selected Publications
- Sin, N.; Kallmerten, J. Synthesis of (2S,3S,8S,9S)-Adda From D-Glucose. Tetrahedron Lett. 1996, 37,5645-5648.
- Cywin, C.L.; Webster, F.X.; Kallmerten, J. Synthesis of (-)-(6R,10R)-Matsuone. Assignment of Relative Stereochemistry to a Pheromone of Matsucoccus Pine Bast Scales. J. Org. Chem. 1991, 56, 2953-2955.
- Coutts, S.J.; Kallmerten J. Synthesis of Antitumor Ansamycins. 2. A Formal Total Synthesis of Macbecin I. Tetrahedron Lett. 1990, 31, 4305-4308.
- Kallmerten J.; Wittman, M.D. Diastereoselective [2,3] Wittig Rearrangement of Tertiary Allylic Ethers. J. Org. Chem. 1988, 53, 4631-4633.
- Kallmerten, J.; Plata, D.J. Total Synthesis of 18-Deoxynargenicin A1. J. Am. Chem. Soc. 1988, 110, 4041-4042.
