SU chemists’ work with small peptide chains may revolutionize study of modern-day enzymes, neurological diseases
Ivan Korendovych's research is subject of major article in 'Nature Chemistry' magazine

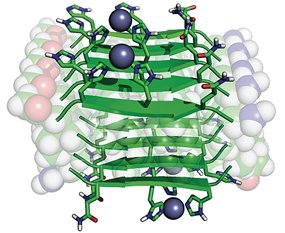
Chemists in Syracuse University’s College of Arts and Sciences have, for the first time, created enzyme-like activity using peptides that are only seven amino acids long.
Their breakthrough, which is the subject a recent article in Nature Chemistry magazine (Macmillan Publishers, 2014), may revolutionize the study of modern-day enzymes, whose chains of amino acids usually number in the hundreds, and of neurological diseases, such as Alzheimer’s, which are usually characterized by small clumps of misshaped proteins called amyloids.
Their finding also supports the theory that amyloid fibrils—strong, highly organized fibers, formed by proteins and peptides—may have predated enzymes and triggered reactions that led to some of the earliest forms of life.
“Enzymes fold into unique three-dimensional structures, which underlie their remarkable catalytic properties and contribute to their large size,” says Ivan V. Korendovych, assistant professor of chemistry at SU, who co-led the study with William DeGrado, professor of pharmaceutical chemistry at the University of California, San Francisco (UCSF). “Our goal was to prove that much shorter peptides can also achieve well-defined conformations through the formation of amyloid fibrils.”
Korendovych and his team designed seven simple peptides, each containing seven amino acids. They then allowed the molecules of each peptide to self-assemble, or spontaneously clump together, to form amyloids. (Zinc, a metal with catalytic properties, was introduced to speed up the reaction.) What they found was that four of the seven peptides catalyzed the hydrolysis of molecules known as esters, compounds that react with water to produce water and acids—a feat not uncommon among certain enzymes.
“It was the first time that a peptide this small self-assembled to produce an enzyme-like catalyst,” says Korendovych, an expert in bioinorganic chemistry, biophysics, and chemical biology. “Our finding suggests that amyloids, whose buildup leads to Alzheimer’s in the brain, may also have served as the blueprint for larger, modern-day enzymes.”
That’s good news for researchers such as Korendovych, who thinks this finding may lead to the development of a new class of synthetic peptide-based catalysts. “The amyloid structures we’ve created may have a more complex biochemistry than we've realized,” he says.
Their breakthrough, which is the subject a recent article in Nature Chemistry magazine (Macmillan Publishers, 2014), may revolutionize the study of modern-day enzymes, whose chains of amino acids usually number in the hundreds, and of neurological diseases, such as Alzheimer’s, which are usually characterized by small clumps of misshaped proteins called amyloids.
Their finding also supports the theory that amyloid fibrils—strong, highly organized fibers, formed by proteins and peptides—may have predated enzymes and triggered reactions that led to some of the earliest forms of life.
“Enzymes fold into unique three-dimensional structures, which underlie their remarkable catalytic properties and contribute to their large size,” says Ivan V. Korendovych, assistant professor of chemistry at SU, who co-led the study with William DeGrado, professor of pharmaceutical chemistry at the University of California, San Francisco (UCSF). “Our goal was to prove that much shorter peptides can also achieve well-defined conformations through the formation of amyloid fibrils.”
Korendovych and his team designed seven simple peptides, each containing seven amino acids. They then allowed the molecules of each peptide to self-assemble, or spontaneously clump together, to form amyloids. (Zinc, a metal with catalytic properties, was introduced to speed up the reaction.) What they found was that four of the seven peptides catalyzed the hydrolysis of molecules known as esters, compounds that react with water to produce water and acids—a feat not uncommon among certain enzymes.
“It was the first time that a peptide this small self-assembled to produce an enzyme-like catalyst,” says Korendovych, an expert in bioinorganic chemistry, biophysics, and chemical biology. “Our finding suggests that amyloids, whose buildup leads to Alzheimer’s in the brain, may also have served as the blueprint for larger, modern-day enzymes.”
That’s good news for researchers such as Korendovych, who thinks this finding may lead to the development of a new class of synthetic peptide-based catalysts. “The amyloid structures we’ve created may have a more complex biochemistry than we've realized,” he says.
There are 20 naturally occurring amino acids, all of which serve as the building blocks of proteins and assist with metabolism.
An enzyme is a type of protein that is composed of at least 100 amino acids and speeds up reactions in a cell.
Korendovych says that, despite an astronomically large number of possible enzymes (each with a different amino acid sequence and three-dimensional shape), only a small number of them actually work.
“Each enzyme has to be an exact fit for its respective substrate,” he says, referring to the molecule with which an enzyme reacts. “Even after millions of years, nature is still testing all the possible combinations of enzymes to determine which ones can catalyze metabolic reactions. Our results make an argument for the design of self-assembling nanostructured catalysts.”
In addition to Korendovych and DeGrado, the article was co-authored by Caroline Rufo, Yurii Moroz, Olesia Moroz, and Tyler Smith, current and former members of Korendovych’s research lab at SU; Jan Stöhr, assistant professor of medicine at UCSF; and Xiaozhen Hu, a postdoctoral researcher at UCSF.
Housed in SU’s Life Sciences Complex, the Department of Chemistry represents teaching and research opportunities in traditional areas, including inorganic, organic, physical and theoretical chemistry, in addition to programs that cross the traditional boundaries between disciplines, such as biomaterials, bioorganic, biophysical, bio-inorganic, and materials chemistry.
An enzyme is a type of protein that is composed of at least 100 amino acids and speeds up reactions in a cell.
Korendovych says that, despite an astronomically large number of possible enzymes (each with a different amino acid sequence and three-dimensional shape), only a small number of them actually work.
“Each enzyme has to be an exact fit for its respective substrate,” he says, referring to the molecule with which an enzyme reacts. “Even after millions of years, nature is still testing all the possible combinations of enzymes to determine which ones can catalyze metabolic reactions. Our results make an argument for the design of self-assembling nanostructured catalysts.”
In addition to Korendovych and DeGrado, the article was co-authored by Caroline Rufo, Yurii Moroz, Olesia Moroz, and Tyler Smith, current and former members of Korendovych’s research lab at SU; Jan Stöhr, assistant professor of medicine at UCSF; and Xiaozhen Hu, a postdoctoral researcher at UCSF.
Housed in SU’s Life Sciences Complex, the Department of Chemistry represents teaching and research opportunities in traditional areas, including inorganic, organic, physical and theoretical chemistry, in addition to programs that cross the traditional boundaries between disciplines, such as biomaterials, bioorganic, biophysical, bio-inorganic, and materials chemistry.
Media Contact
Rob Enslin
