Shielding the Cell
A&S Professor Uncovers 'Cage' of Proteins

Imagine you are reading a book and as you turn the page you suddenly feel a sharp pain from your finger – you have a papercut. You will probably just put on a Band-Aid™ and continue reading. Little do you know that your body has already gone into repair mode. Cells called fibroblasts are rushing to the wound site to begin the healing process. It’s a difficult journey for the cells as they pass through areas of dense tissue, but luckily the fibroblast cells have a form of protection called vimentin. New functions of this cage-like network within the cell were recently discovered by a team of scholars that included Alison Patteson, assistant professor of physics in Syracuse University’s College of Arts and Sciences (A&S).
Known as vimentin intermediate filaments, the network of proteins protects the nucleus against deformation, rupture and DNA damage during migration, often present in processes like wound healing.
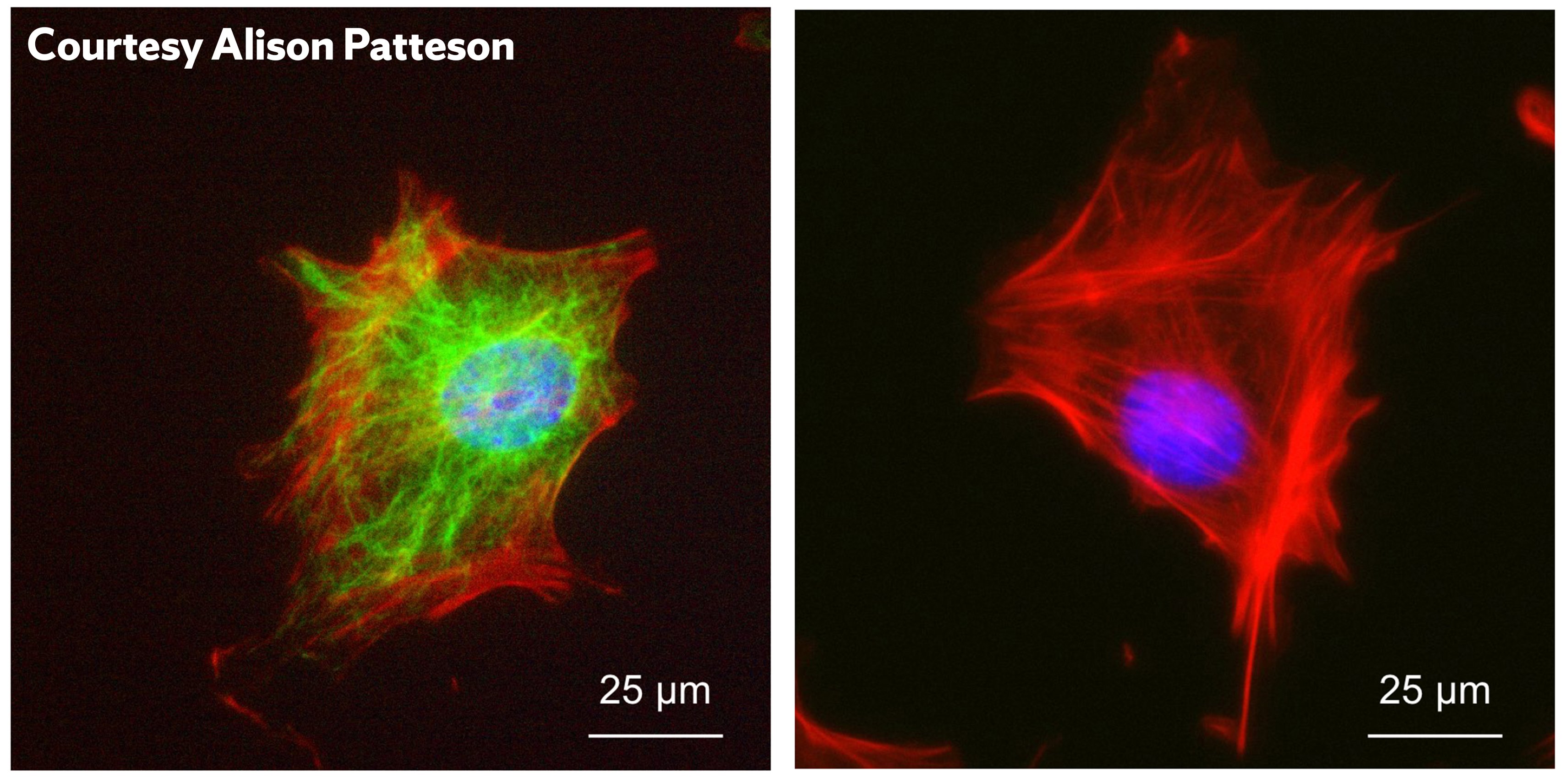
“During wound healing, your cells have to transition from being stationary skin cells to being migratory cells,” Patteson says. “When this occurs, they start expressing a protein called vimentin. Cells tend to form this when they are going to migrate in the body.”
Research by Patteson showed that when the cage-like protein was present in cells, there was 50 percent less DNA damage in the nuclei. Cells without the vimentin protein displayed ruptures, likely to cause DNA damage. A study conducted on mice showed that mice without the protein had impaired wound healing capabilities, providing direct evidence that the protein helps protect the cells during the repair process. The cutting-edge research was published in the Journal of Cell Biology.
“For me, it’s the first time I’m cross pollinating into a different field,” she says. For the physics professor, having results published in a prestigious biology journal is exactly the type of interdisciplinary work she hoped to be involved in as part of Syracuse University’s BioInspired Institute.
BioInspired aims to unite faculty from different schools and colleges within the university who have related research interests. BioInspired supports research to address major challenges in health, medicine and materials innovation.
Patteson’s research was a collaboration with Robert Goldman from the Department of Cell and Molecular Biology at the Feinberg School of Medicine, Northwestern University, and principal investigator Paul Janmey from the Institute for Medicine and Engineering at the University of Pennsylvania.
In the past, the role of vimentin remained largely unclear, but their results play an important role in determining the fate of the cell and protection of its genetic material. Now that researchers know more about the protein vimentin, their next step is understanding both how it is formed and how it protects the nucleus from damage during cell migration.
“Our ultimate goal is to figure out how cells move in environments like the body,” Patteson says. “A common method for studying cells is to use petri dishes which are flat, nothing like inside the body.”
Understanding the role of vimentin will shed light on exactly how cells move through tissues. Upcoming research will focus on collective cell motility (how cells move in large groups), like during cancer growth. “In many types of cancers, this protein is abnormally expressed at high levels,” Patteson says. “It’s used as a marker for aggressive tumor growth and poor patient prognosis.” Figuring out how to disrupt this protein and stop its assembly could potentially lead to a future breakthrough in the fight against cancer.
